Carbon capture
Carbon capture and storage (also known as carbon sequestration) is a method of mitigating the CO2 emitted from the combustion of fossil fuels. It is a way of storing the gas before it is allowed to enter the atmosphere and more recently it also refers to scrubbing CO2 from ambient air, for example using carbon scrubbers or converting it into a useful product via artificial photosynthesis.
Carbon storage
Various methods have been suggested for storing CO2, which include storing it in existing geographical formations, deep in the oceans or in the form of mineral carbonates. These will be discussed in more detail below.
Geological storage
This method involves injecting CO2 into underground geological formations including old oil fields, gas fields, saline formations and old coal mines. By injecting CO2 into declining oil fields, this can force more oil out of the well and the cost of storing the gas can be partially offset by the sale of the extra oil recovered. However they do have a limited capacity and locations are also finite. In the case of mines, CO2 attaches itself to the surface of the coal which in turn releases methane which can be sold to offset the cost of storing the gas in the first place. However like pushing out and using the extra oil, burning the methane will produce more CO2. It also is important that the coal field is not over permeable otherwise the gas could leak. However good locations are available in all the geographical locations where the gas could be stored for millions of years.
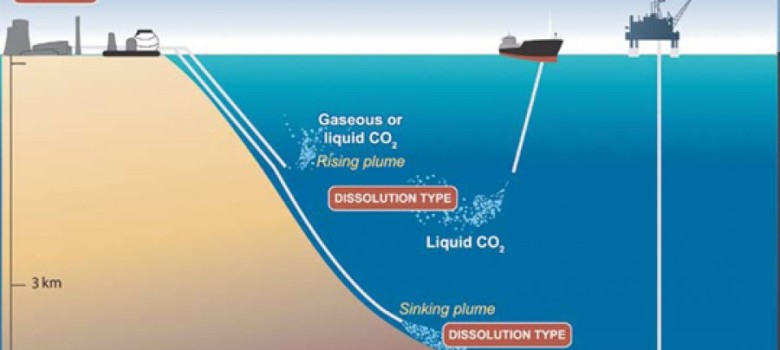
Oceanic storage
There are two major methods of ocean storage; the first is pumping the gas down to depths of 1-3km and letting the gas rise, where it will dissolve into the seawater, this will slowly come out of the water back into the atmosphere. The other method is pumping it down further than 3km, and at this depth the pressure will cause the CO2 to liquefy, and as this liquid is denser than the sea water it will fall to the bottom of the sea and form non reactive pools of liquid CO2. Both these methods have potential but the effect of additional quantities of the gas may well upset ecosystems, so more research needs to be completed in this field.
Mineral storage
This method involved reacting the CO2 with metal oxides, forming metal carbonates. This reaction requires heat, however the metal oxides needed for the reaction are plentiful (such as Magnesium) and the produced carbonates are very stable so they will hold on to the gas for millennia. The major drawback is the heat required for the reaction, so research is ongoing to make this a more economically viable option.










No Comments yet! Be the first one.